Lymphocytes are probably the most investigated cell type in immunological research.
What number of naive T cells does the donor have? Which of those are specific for
antigen A? What does their cytokine profile look like? What ratio of CD4+ and
CD8+ the donor has.
We have to distinguish the CD4+ and the CD8+ T lymphocytes. The CD4s are playing
a role in the humoral immunity. And the CD8s are 'responsible for' the cellular
immunity. In relation to these functions people look at their phenotypic and
cytokine profiles.
In most applications the activation of T cells requires the presence of
antigen presenting cells (APC), like B cells and monocytes. Especially when one
would like to look at antigen specific responses. In some cases researchers use
polyclonal stimuli like PMA/ionomycin or lectins, like PHA or ConA.
What do we look
for in the CD4 T lymphocyte subset?
CD4s can be divided in a memory population, the cells that already have been
activated by their antigen, and the naive population, the cells that didn't
meet their antigen yet. By staining the lymphocytes with CD4, the naive or memory marker (CD45RA/CD45RO in human) and one or more
cytokines it is possible to look at cytokine profiles. When several parameters
are evaluated we would suggest to
use at least the fluorescence 2 channel for the cytokine. Use the first for the
CD4 and the third for the naive/memory marker. And the fourth channel for an
additional cytokine or (most often) switch the first and the fourth channel. To
stimulate the cells, otherwise the cells
will produce very low or no cytokines, stimulation for 6 hours with a
combination of phorbol 12-myristate 13-acetate (PMA) and ionomycin will do best. The last 4 - 5 hours you should add a transport inhibitor
(see protocols) to prevent secretion of the
cytokines.
In some protocols the expected percentage of positive cells is very low, for instance
antigen specific reactions to house dust mite. There
are different ways to look at these samples. One of them is to evaluate (only)
the activated cells by using an activation marker.
On of the earliest changes in the phenotype of the activated T cell is the
expression of CD69. Which expression is the highest at 8 - 10 hours. The
advantage of this marker is the fact that it will be expressed at high levels
for 2 days by (almost) all activated cells. In a later phase of the response markers
like HLA-dr and CD25 (the IL-2 receptor) are expressed.
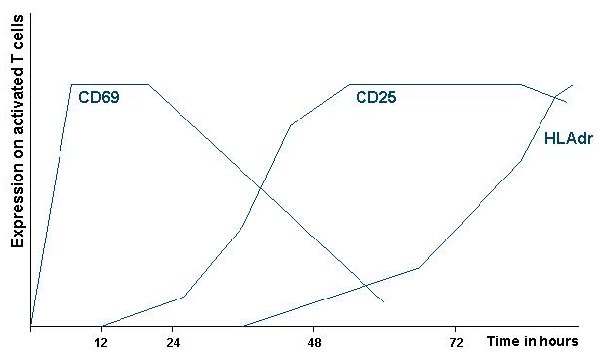
Figure.1. Kinetics of the expression of T lymphocyte activation
markers CD69, CD25 and HLAdr. Double staining with these markers makes it
possible to look for low numbers of activated cells (see paragraph on antigen
specific staining of T lymphocytes).
Distinction between Th1 and Th2 CD4+ T lymphocytes.
You could also look at the results of skewing CD4+ T lymphocytes towards the T
helper (Th)- 1 or Th2 phenotype. These to extremes of a large spectrum of
different helper cells are characterized by the cytokines they produce. The T
cell belonging to the Th1 phenotype produces high levels of gamma interferon
(IFNy) and no interleukin-4 (IL-4) or IL-5. The T lymphocyte belonging to the Th2
side of the spectrum produces high levels of IL4 and IL5 and no IFNy.
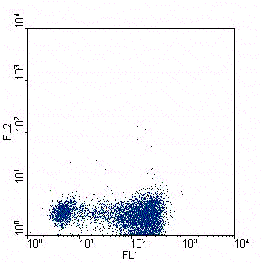
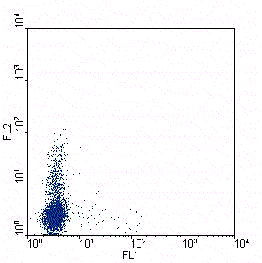
Figure.2.A. Human naive T lymphocytes have been skewed towards a Th1 (left)
or a Th2 phenotype (right). When the cells where resting after the skewing
protocol they where stimulated for 6 hours with PMA/ionomycin. During the last
4 hours brefeldin A was added. Samples where stained with a anti-IFNy-FITC
(X-axes) and anti-IL-4-PE (Y-axes) to detect the cytokines and counterstained with
anti-CD4-PerCP to be able to exclude dead cells. Only CD4+ cells are shown in the figure.
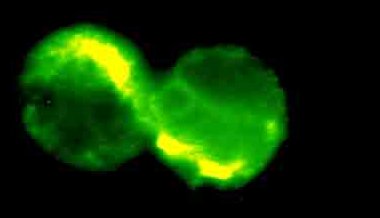
Figure.2.B. Fluorescence microscope image of two IL-2 positive human
CD4+ T lymphocytes. Cells where taken from the same protocol as used for
fig.1.
Antigen specific stimulation of T lymphocytes.
It is also possible to look at antigen specific T lymphocytes from the blood. These
protocols look straight forward but lots of researchers already tried with
disappointing results.
But S.L.Waldrop (see literature) shows (using the
adapted protocol described by L.J.Picker) clearly the cytomegalovirus (CMV)
reacting T cells from periferal blood. Looking at the results it is clear that
the percentage of (CMV) specific T lymphocytes is not very high. Percentages
lower than 1% cytokine positive cells in the CD4+/CD69+ population are not
unusual. This is why 45,000 - 50,000 events in the CD4+ gate where evaluated.
And the researchers look at the activated T lymphocytes by using the early
activation marker CD69. Donors prone to be (or were) positive for the virus show
higher percentages.
Taken from the article Waldrop S.L, et al. J.Clin.Invest.
Vol.99. Number 7. 1997. 1739 - 1750.
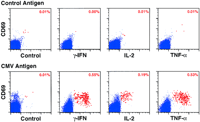
Fig. 1. Detection and characterization of CMV-specific CD4+ T cells by multiparameter flow cytometric detection of CMV Ag-induced CD69 upregulation and
cytokine synthesis. (A, top) Normal PBMC were stimulated with CMV Ag versus control Ag preparations for 6 h in the presence of the secretion inhibitor Brefeldin
A for the last 5 h, and examined as described in Methods for their correlated expression of intracellular cytokine (-IFN, IL-2, TNF- versus Control) versus the
activation antigen CD69 versus CD4. 48,000 events, gated on viable CD4+ lymphocytes, are shown in each plot. Events in the cytokine+, CD69+ "response" region
of the profiles are colored red (with the percentage of these cells given in the upper right hand corner), whereas events in the "nonresponding" region are colored blue.
The responses shown for control Ag (top) were identical to that observed in the absence of any added Ag (Brefeldin A alone). The experiment shown is
representative of > 10 independent experiments.
What about CD8+ T lymphocytes?
The same protocols as used for the
CD4+ T cells can be used for the CD8+ cells. The cytokine patterns for these
cells are different though. And CD8+ are producing other proteins like granzyms
and perforins. It has been shown that also these proteins can be detected in the
CD8+ T cell.
What cytokines to look for and over which time interval?
As already mentioned; that is a very hard question to answer. Every species,
every cell type and every situation is different. We can only suggest to find
articles from similar research and try to find the right time point. Lymphocytes
produce IL-2 and IFNy over longer period (up to 36 hours), IL4 and IL-5 are
produced in the onset of the reaction for only a short period (up to 12 hours)
and a cytokine like IL-10 is produced in the later part of the reaction.
In general lymphocytes are stimulated with PMA/ionomycin for 6 hours (last 5 in
the presence of a transport inhibitor) or over night with a super antigen or
PMA/CD3 to detect those cytokines like IL-10 (last 12 hours in the presence of a
transport inhibitor).
Do B lymphocytes differ?
No they don't. It is possible to stimulate these cells with PMA/ionomycin to
look at cytokine production. But it is also possible to look at the type of
antibody the B cells (plasma cells) produce and that way look how heterogeneous
a population of cells is.
Cross linking certain receptors/molecules on the membrane to activate the cells
might be tricky. When a secondary antibody has to be used to detect one of the
parameters in the cytometer this may raise the background. When all antibodies
are directly conjugated with a fluorochrome (or biotin) this problem will be
minor.