More difficult than lymphocytes or monocytes are the dendritic cells from the
blood or the monocyte derived dendritic cells (moDC's). The dendritic cells from the periferal blood are usually very low in number,
<1%. These cells can be isolated (enriched) by many different (magnetic separation
based) kits. But there are also other dendritic cells like dendritic cells from the skin, Langerhans
cells. Their number is often low and hypothesis often ask to determine more
parameters than possible. This often means that we have to perform a multi-fluorochrome
(parameter) labeling. This might not only be a problem with respect to the staining of the intracellular target but also
to the phenotypic markers since most of these cell types do not have a specific lineage
marker.
Monocyte derived dendritic cells.
(Human) monocyte derived dendritic cells (moDC) are at this moment the easiest
accessible 'source' of dendritic cells. These cells are generated in 6 days from
monocyte cultures in the presence of GM-CSF and IL-4. At day 6 they have lost
CD14 (monocyte lineage marker) and gained CD1a. "Classical" stimuli like lipopolysaccharide (LPS) can
stimulate the (immature) moDC's to produce factors like IL-6, IL-8 and IL-12(p40
and p70).
Often the hypothesis is related to IL-12. IL-12 is regulated in the
production of one of the two subunits, the p35. The other one is produced
abundantly and shared by IL-23. Only the heterodimer p70 is biological active and for that matter
most interesting to measure. There are only limited Ab's available that are
specific for this molecule. The 20C2 is one that is specific for the heterodimer.
The intracellular detection of the biological IL-12p70 is still difficult. The cells have a reasonable high autofluorescence and the
subunits are 'put together' at the very last moment. Since monensin is activating
monocytes it is wise to use BFA in this protocol. One thing is sure; IFNy has to
be added to the culture when the cells are stimulated to find detectable IL-12p70.
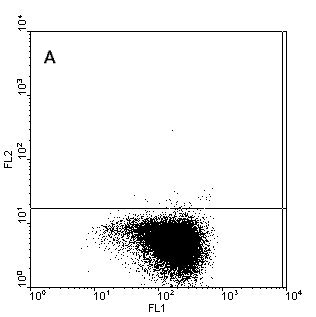
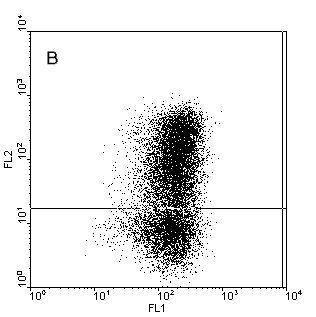
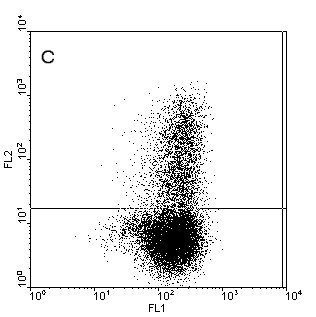
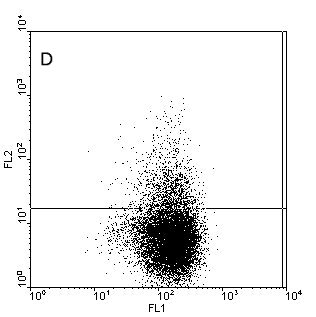
Figure.1. Intracellular staining of human monocyte derived
dendritic cells (moDC) for IL-12p40 (b), IL12-p70 (c) and TNFa (d) after stimulation with
CD40-ligand in the presence of IFNy or unstimulated and stained for IL-12p40 (a). Samples where
stained with anti-CD1a-FITC (X-axes) and the cytokines in PE (Y-axes) to detect the cytokines and
counterstained with anti-HLAdr-PerCP. Only HLAdr+ cells are shown in
the figure.
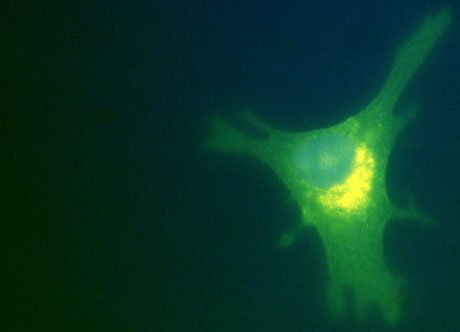
Figure.2. Confocal image of an intracellular staining of human monocyte derived
dendritic cell for IL6 after 6 hours stimulation with PMA/ionomycin. Cells
have been plated in chamber slides one day before stimulation. Samples where
stained with anti-CD1a-FITC (green) and anti-IL6-PE (orange (+
the green gives yellow signal)) to detect the cytokine and
counterstained with HOECHST to show the nucleolus (blue).
Presentation on intracellular staining of dendritic
cells.